Introduction – NAG Kit How it Works
Until now the laboratory measurement of NAG (NAGase) activities has been complex. The early fluorimetric assays posed standardisation problems whilst the early chromogenic substrates based on p-nitrophenol, due to their yellow colour, had very high blanks and thus a low sensitivity. More recently two new chromogenic substrates have been developed for the urinary NAG assay, namely methylcresol phenolphthalein glucosaminide and omega-nitrostyryl glucosaminide. The former has a high extinction coefficient when the chromophore is released but the substrate itself has a high absorption in the visible region so the colour change is less apparent.
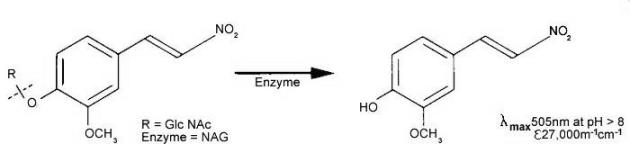
Figure 1. Catalytic reaction of NAG on MNP-GlcNAc
Substrate of choice
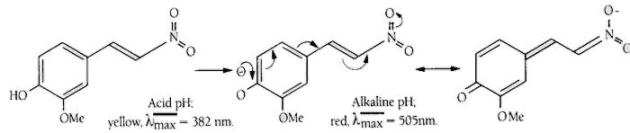
The latter, namely 2-methoxy-4-(2-nitrovinyl)-phenyl-glucosaminide is pale yellow in acid solution and the released chromophoric phenol is red coloured and has a high extinction coefficient at 505nm in alkaline solution. This substrate has proved to be highly sensitive and is the basis of a NAG test pack researched and developed by scientists at King’s College London.
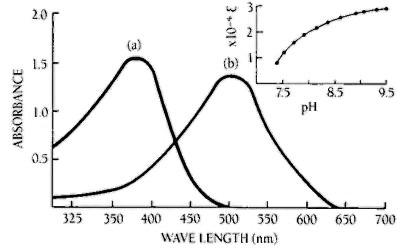
Figure 2. Effect of pH on the absorption maximum and the molar extinction coefficient of MNP
Absorption of MNP at concentrations (a) 0.1 mM in buffer of pH 5.0, (b) 0.05 mM in buffer of pH 9.5. The insert shows the increase of molar extinction coefficient (Lmol-1cm-1) with increasing pH at 505 nm.
The package
Each test pack contains: Bottle N- a solution of the substrate, Bottle A- a mixture of crystaline buffer salts and Bottle G- a ready to use solution of colour development buffer, together with two vials of Calibrant and a kit instruction sheet. Additional calibrants and NAG enzyme controls can be purchased separately. (See NAG controls and calibrants).
The principle
2-Methoxy-4-(2-nitrovinyl)-phenyl 2-acetamido-2-deoxy-β-D-glucopyranoside (MNP-Glc-NAc) is hydrolysed by N-Acetyl-β-D-Glucosaminidase (NAG) with the release of 2-methoxy-4-(2-nitrovinyl) phenol which, on addition of alkaline buffer, produces a colour which can be measured at 505nm.
Figure 3. Principle of the colour change in the NAG reaction
Reference values: urine
If timed urine samples are to be used for this test the optimum collection period is 24 hours, although such collections may be difficult to administer. It required, a first or timed morning sample, or daytime sample of suitable collection time can also be used. Random samples may be corrected for urine volume by factoring with the creatinine value for the sample as follows:
Corrected activity = NAG value (μmol/h/L) ÷ creatinine (mM)
E.g. Corrected activity = 152 (μmol/h/L) / 10.4 (mM) = 14.6 (μmol/h/mmol creatinine)
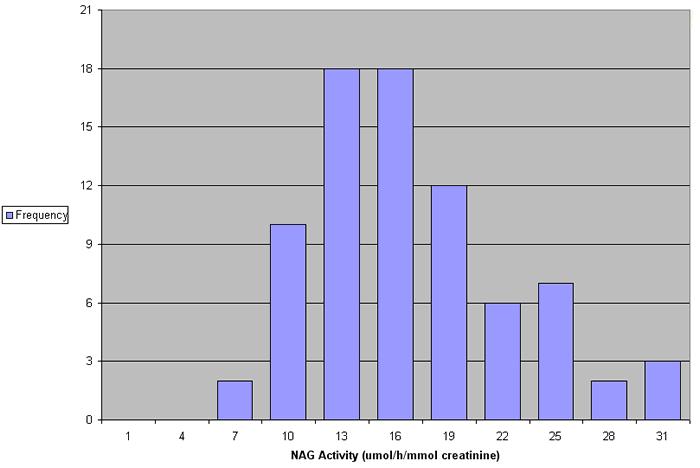
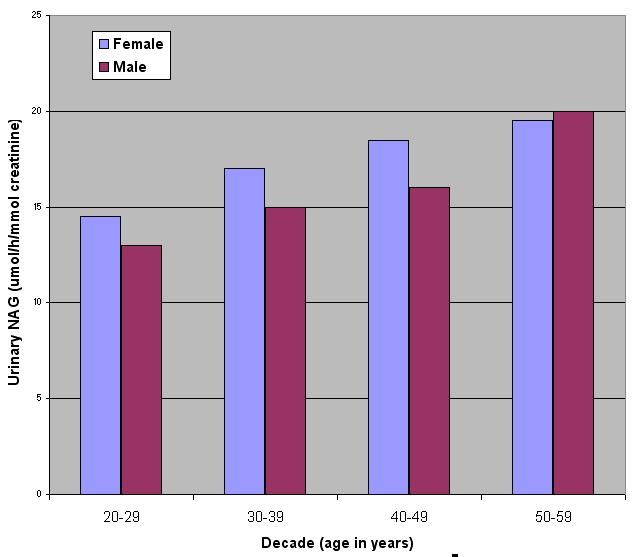
In a study of 78 normal individuals (aged 20-59) NAG values ranged between 6.8 and 30.8 µmol/h/mmol creatinine (see Figure 4). No significant differences were found in the excretion of NAG in males and females (see Figure 5).
Figure 4. NAG values in 78 normal individuals
Figure 5. NAG activities across different age groups
Observed NAG values are higher in children under 10 years and, because of the fall in muscle mass, in individuals over 60 years, and should therefore be separately determined. Stable renal transplant patients excrete NAG at a higher rate than in normals.
NAG values: serum
NAG values observed in serum samples are considerably higher than those found in urine: in addition NAG values are known to rise very considerably during pregnancy.
Modifications
Simple modifications enable this assay to be performed on most discrete and centrifugal-type analysers. Instructions are also available to assist isoenzyme studies which can be carried out using the same test packs.